What to Expect
Calling all champions of innovation
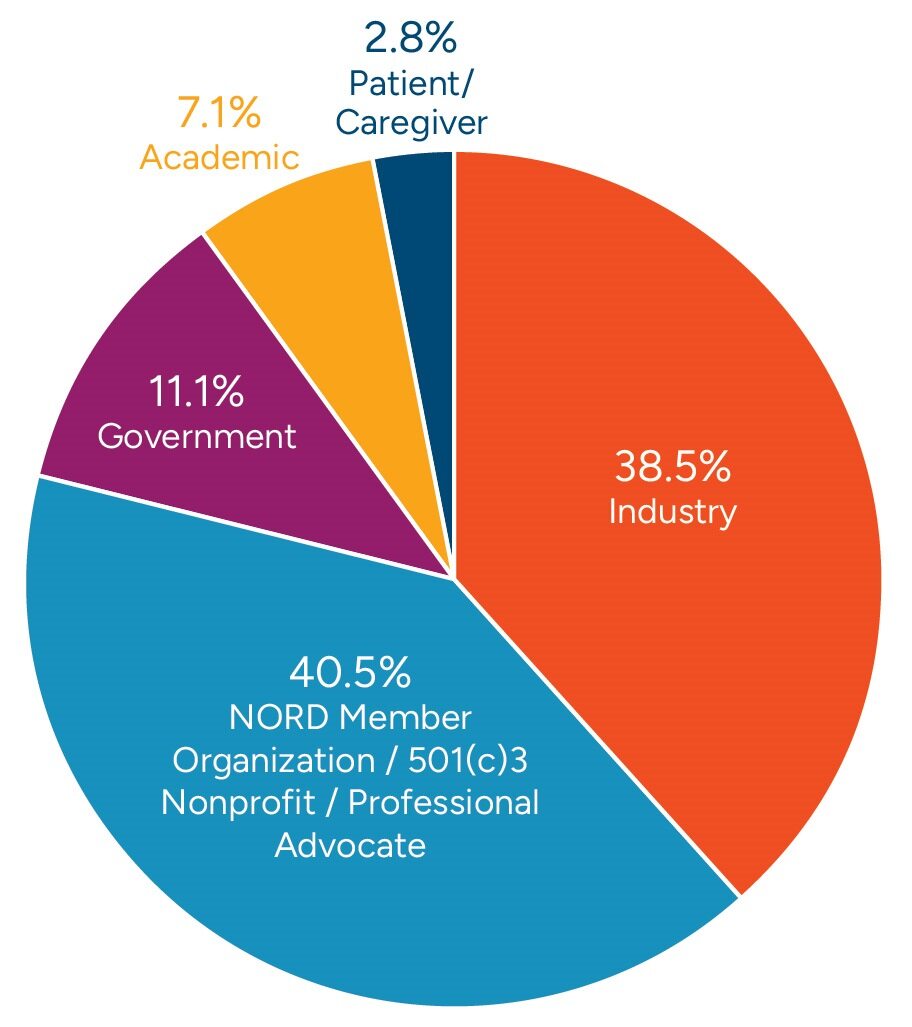
Join over 900 patient leaders, researchers, healthcare advocates, pharma and biotech innovators, government agencies, and others to tackle the most pressing issues facing the rare disease community.
Pharma and Biotech
-
Hear the latest in orphan product development from top FDA leaders
-
Explore new business ventures
-
Connect with patient advocates
Government Officials and Regulators
-
Understand the policy and regulatory impact on orphan products
-
Learn about scientific advancement in the rare disease community
CROS and Solutions Companies
-
Connect with clients
-
Cultivate prospects who require your expertise and services to develop pipelines, conduct trials and studies, and pursue commercialization
Patient Advocacy Leaders
-
Understand the policy and regulatory impact on orphan products
-
Learn about scientific advancement in the rare disease community
Researchers and Scientists
-
Engage with cutting-edge research and innovative solutions
-
Network with peers and explore collaboration opportunities
